Background
According to a recent study by U.S. CDC, more than 65% of individuals infected with hepatitis C virus (HCV), and who have continuous insurance coverage (private, Medicaid or Medicare), did not initiate treatment within one year of being diagnosed.1 This is a disturbing statistic given that current HCV treatments are curative in more than 95% of cases, they are oral treatments, and have a short duration of treatment.
U.S. CDC analyses also showed that for people living with hepatitis B virus (HBV) infection, lack of awareness of HBV infection, poor access to testing, and linkage to care remains an issue.2 Monitoring and treatment, while not curative, greatly reduce the risk of cirrhosis and hepatocellular carcinoma (HCC).
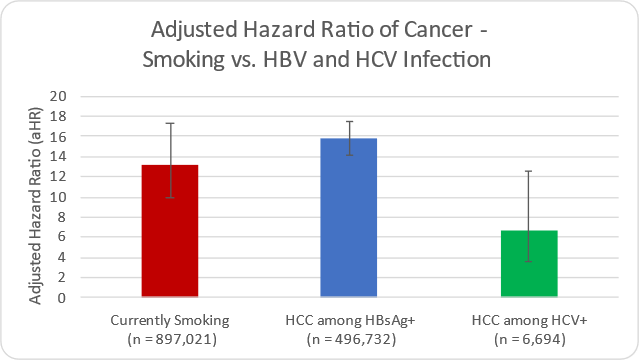
Long-term infection with Hepatitis B and C viruses (HBV & HCV) causes chronic inflammation of the liver and ultimately liver cancer. The risk of developing cancer in individuals infected with HBV and HCV is similar to the risk of cancer in someone who currently smokes.3 In addition, treatment of those infected with HBV or HCV can reduce the risk of cancer by more than 75%.4
HCV and HBV infections are also associated with increased risk of non-Hodgkin’s lymphoma, diabetes, insulin resistance, glomerulonephritis, renal insufficiency, fatigue, cognitive impairment, depression, impaired quality of life, polyarthritis, fibromyalgia, and cardiovascular disorders (i.e. stroke, ischemic heart disease).5
A study by researchers at Mount Sinai’s Icahn School of Medicine showed that reaching out to individuals who have been diagnosed but untreated can result in 31% linkage to care.6 This groundbreaking study demonstrated that it is possible to reengage infected individual who were diagnosed but lost to follow up. Expanded treatment is required for the U.S. to eliminate viral hepatitis as a public threat before 2030.7
Relink Grant Program Objective
CDA Foundation has received an eight-million-dollar grant from Gilead Sciences to distribute to sub-grantees in 2023–2025.
The objective of the ReLink program is to provide grants to find diagnosed-but-untreated HCV and HBV infected individuals and help them get linked to care. The program is treatment agnostic and Gilead will not have any influence on the design, implementation, and the RFP process including reviewing and evaluating of proposals. Diagnosed patients can go on any treatment recommended by their doctor.
Eligibility Criteria
The following type of organizations are eligible to apply for Relink grants:
- Tax-exempt organizations serving the U.S. population.
- Tax-exempt Healthcare institutions (hospitals, clinics, universities).
- Non-profits working with patients.
- Public Health Agencies.
Expenses Not Covered by the Grant
- Medications or purchasing of medications.
- Direct medical expenses, including lab expenses.
- Existing deficits of grantee.
- Biomedical research, clinical research, or clinical trials.
- Projects that directly influence or advance pharmaceutical companies’ business, including purchase, utilization, prescribing, formulary position, pricing, reimbursement, referral, recommendation or payment for products.
- Individuals, individual health care providers, or physician group practices.
- Medical care – this should already be in place.
- Events or programs that have already occurred.
- Government lobbying activities.
Ineligible States and Territories
The Relink grants will be available to all states/territories that provide parity access to HCV and HBV medicines. The following states/territories will be excluded from the grants since they have preferential prescription agreements in place:
- Louisiana
- Michigan
- Minnesota
- Missouri
- Montana
- Oklahoma
- Puerto Rico
- Texas
- Washington
- Washington, DC
Key Dates
For Round Four, the following dates will apply:
| RFP release date | September 1st, 2025* |
| Proposal due date | November 1st, 2025 (23:59 MT) |
| Review proposals by advisory board | November 4th – November 16th, 2025 |
| Proposal approval notification date | December 16th, 2025 |
| Contracts completed and distribution of the grants | January 15th, 2026 |
*An early submission window is now open for Departments of Health at the city, county or state level. More here.
Contact Us
The request for proposal (RFP) is now available for download. For more information, please contact us at relink@cdafound.org.
References:
- Thompson WW, Symum H, Sandul A, et al. Vital Signs: Hepatitis C Treatment Among Insured Adults — United States, 2019–2020. MMWR Morb Mortal Wkly Rep 2022;71:1011-1017. DOI: http://dx.doi.org/10.15585/mmwr.mm7132e1.
- US Department of Health and Human Services. Viral Hepatitis National Strategic Plan: A Roadmap to Elimination for the United States, 2021-2025.
- Razavi H, Estes C, et. al. Risk of developing cancer – comparison of HBV, HCV, and smoking. EASL 2023.
- Morgan RL, Baack B, Smith BD, Yartel A, Pitasi M, Falck-Ytter Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: a meta-analysis of observational studies. Ann Intern Med. 2013 Mar 5;158(5 Pt 1):329-37. doi: 10.7326/0003-4819-158-5-201303050-00005. PMID: 23460056.
- Cacoub P, Comarmond C et. al. Extrahepatic manifestations of chronic hepatitis C virus infection. Ther Adv Infect Dis (2016) 3(1) 3-14.
- Wyatt B, Perumalswami PV, Mageras A, et al. A Digital Case-Finding Algorithm for Diagnosed but Untreated Hepatitis C: A Tool for Increasing Linkage to Treatment and Cure. Hepatology 2021; 74(6): 2974-87.
- Strom BL, Buckley GJ, eds. A national strategy for the elimination of hepatitis B and C: phase two report. Washington, DC: National Academies Press; 2017. PMID:28737845 https://doi.org/10.17226/24731
The Relink program has been made possible through a grant by Gilead Sciences.